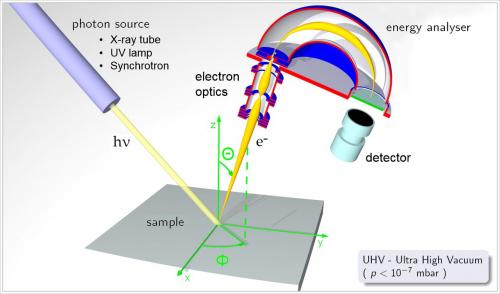
 X-ray Photoelectron Spectroscopy (XPS) or Electron Spectroscopy for Chemical Analysis (ESCA) is a technique which analyzes the elements constituting the sample surface, its composition, and chemical bonding state by irradiating x-rays on the sample surface, and measuring the kinetic energy of the photoelectrons emitted from the sample surface. XPS instrument using Al Kα rays can generally obtain information on elements within a few nms of the sample surface.
X-ray Photoelectron Spectroscopy (XPS) or Electron Spectroscopy for Chemical Analysis (ESCA) is a technique which analyzes the elements constituting the sample surface, its composition, and chemical bonding state by irradiating x-rays on the sample surface, and measuring the kinetic energy of the photoelectrons emitted from the sample surface. XPS instrument using Al Kα rays can generally obtain information on elements within a few nms of the sample surface.
Additionally, the change in bond energy (chemical shift) caused by the electron state surrounding the atoms to be analyzed, such as atomic valence charges and interatomic distances, tend to be greater than the chemical shift observed in AES, which makes the relative ease with which the state of chemical bonds can be identified another advantage of XPS.
