In photoelectron spectroscopy (PES) recorded electrons are emitted when the surface is irradiated with photons. The technique is based on the photoelectric effect. The atoms in solids are ionized and the electrons with binding energies (Eb) absorb photons with energy hν (1486.6 eV for Al Ka) and escape from the solid body with kinetic energy Ek:
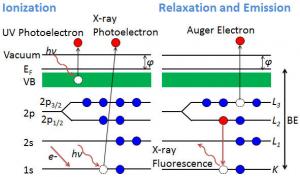
where the material work function
Ø = Evacuum – EF
Following ionization with photoelectron emission from core levels or valence band, the atom will release energy by Auger electron emission or X-ray fluorescence. The Ek is measured using an electron energy analyzer which generates a spectrum with a series of photoelectron peaks. The Eb of the peaks are characteristic of each element.
- The peak areas can be used (with appropriate sensitivity factors) to determine the composition of the materials surface.
- The peak shape and Eb can be slightly altered by the chemical state of the emitting atom providing chemical bonding information. The XPS is not sensitive to hydrogen or helium, but can detect all other elements.
Depending on incident photon energy (wavelength), PES is usually divided in two types:
- X-ray photoelectron spectroscopy (XPS) with X-rays photons between 100 eV-10 keV (soft X-rays, wavelengths: 10 - 0.1 nm) is applied to probe deep core levels;
- ultraviolet photoelectron spectroscopy (UPS) which uses photons in the ultraviolet spectral range of 10-50 eV (wavelengths: 100 to 25 nm) is applied to study the valence and conduction bands and to measure work function.